Using Iron in Soil, Hydroponics & Aquaponics
Iron is needed in plants for several functions. Iron is used in chlorophyll production, it acts as an oxygen carrier, and it is involved in chemical reactions for cell division and growth. Because iron is involved in chlorophyll production one visual sign of iron application in plants is a quick greening. Iron is used extensively in turfgrass and sod production for this purpose.
Iron deficiency also may be a problem in certain lawns, shrubs and ornamentals. In terms of crops, peach trees, sorghum, corn, potatoes, sorghum, sudan, sorghum-sudan hybrids and pinto beans are most severely affected. Wheat and alfalfa are the least sensitive.
Iron Deficiency
Iron-deficient fields, when viewed from a distance, have irregularly shaped yellow areas. Because iron is not translocated or moved in the plant, deficiency symptoms appear on the new growth first. Iron deficiency on individual plants is characterized by yellow leaves with dark green veins (aka interveinal chlorosis). On corn and sorghum this gives the plants a definite striped appearance. If the condition is severe the whole plant may turn very light yellow or even white. In many cases, where moderate deficiencies occur early in the season, plants tend to recover later.
Forms of Iron
There are several forms of iron that can be purchased and each has its pros and cons depending on the system in which it is used. Iron deficiency is associated with high pH and excessive calcium carbonate in the soil so the possibility of lowering the soil pH to correct the problem has been suggested. The rate of sulfur necessary to accomplish this on calcareous soils is not economically feasible (1 pound sulfur for every 3 pounds of CaCO3). Iron oxide fertilizers are highly water insoluble. Fertilizer containing this form of iron will not be available to plants. Iron chelates are available that will correct this iron deficiency.
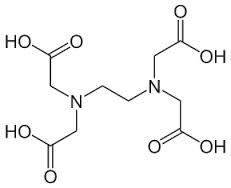
What is a Chelate?
Chelation is a term that describes an encapsulation process. A mineral, such as calcium for example, is reacted with another material to form a protective shell around the desired mineral or metal (in this case calcium). The word chelate derives from the Greek word "chel," meaning a crab's claw, and refers to the pincer-like manner in which the mineral is bound. Some chelating chemicals are shaped like a letter "C" and surround the mineral with just one molecule. This type of binding is called a "complex." When two molecules of the same material surround a mineral it is known as a chelate. It is important to note that some minerals, like boron or molybdenum, have only one chemical bond and are thus limited to forming only a complex. Strictly speaking, boron and molybdenum cannot be chelated minerals but they are often presented and sold in the market as chelated minerals.
Chelates need a "glue" to hold the protective shell in place. Some chelates use sodium for this purpose, but adding sodium can be detrimental to plants. In general, the amino acid chelates use organic acids like citric acid. There is an important distinction to be made here about the use of the word organic. In chemistry the term organic means the material contains carbon. In the organic plants world the term organic refers to plants produced without pesticides, synthetic substances, sewage products and other restrictions.
Chelates are molecules with a neutral charge which is very important. Micronutrients normally have an electrical charge on them. For example, calcium and magnesium are both +2 charge. Soil is generally negative in charge, especially clay soils. This means calcium and magnesium will likely react with the soil and be insoluble and not available for plants to use. Moreover, since they are the same charge, calcium and magnesium compete with each other for entry into the plant. Pores on the leaves of plants (also known as stomates) are negatively charged, so positively charged molecules trying to penetrate the plant get tied up at the stomate entrance (leaf pores) thus slowing absorption. But the interaction with chelates is very different. The neutral charge of chelated minerals allows them to enter the stomates unimpeded. Research into nutrient uptake has shown some materials applied to leaves do not enter the tissues but instead remain stuck to the leaf surface like house paint. Subsequent chemical analysis of these tissues would show similar nutrient levels as those tissues that had nutrient penetration. In light of this oversight researchers now apply nutrients to the leaves and then analyze the fruits to measure the amount of nutrient movement inside the plant.
There are several types of chelates. One of the most common forms is ethylene diamine tetraacetic acid (EDTA), which has been on the market for years. EDTA is a large synthetic molecule which binds very tightly to minerals and resists chemical interactions — desirable characteristics for chelates used in the soil. But this strong bonding characteristic can be a negative attribute once EDTA is in the plant. EDTA binds tightly. In fact, when people have heavy-metal poisoning EDTA is injected into their bloodstream to chelate the metals and filter them out. Obviously patients do not want EDTA releasing heavy metals back into their body prematurely. In addition, EDTA can solve one plant nutrient deficiency and at the same time cause another. EDTA has something of a separation anxiety; it must always hold on to something. For example, iron EDTA will cure iron deficiency in plants, but in order for the EDTA to release the iron it must hold onto something else. Often EDTA will take up manganese in order to release the iron, thus causing a manganese deficiency. This exchange can be frustrating because manganese-deficiency symptoms are very similar to iron-deficiency symptoms. Furthermore, EDTA is known to take calcium from cell walls in both plants and people. For this reason people put on EDTA are often instructed to take calcium supplements as well. Plants losing calcium in this manner (primarily from their cell walls) visually manifest the loss as wilting. EDTA is also a high pH product because it must contain sodium to be soluble in water. Sodium is not desired as it can cause salt damage. And, since EDTA is a high pH product it has a propensity to burn plants easier than other products.
Another category of chelate is the amino acid chelates. There are 20 amino acids. Amino acids are the building blocks of protein. Amino acids are moderately strong chelating agents. Once inside the plant the mineral is released and the leftover amino acids that formed the protective shell are used by the plant as a source of water soluble nitrogen. After all, amino acids are building blocks in cell machinery. Everything is used, nothing is lost. Conversely, EDTA is a synthetic molecule, and plants do not naturally use EDTA. It's sort of like trans-fat; the human body doesn't know what to do with it. Amino acid chelates are generally systemic in the plant, meaning they move and travel to where they are needed. They can do this because amino acids are recognized by the plant as building blocks and are used in nearly every tissue in the plant.
Because chelates enter the plant easily they are extremely useful for quickly correcting nutrient deficiencies. As a rule chelates are very safe for the plant. The amino acid chelates (glycine chelates included) require large amounts of product to be applied in order to be toxic to plants. But care must be taken to avoid phytotoxicity or burning of plant tissues with EDTA.
Amino acid chelated iron is made from iron sulfate which qualifies the product for organic certification. However, the iron sulfate must come from a mined source. Iron sulfate made with sulfuric acid is prohibited for organic certification. From an efficacy standpoint, the plant does not know the difference, iron sulfate is iron sulfate.
Using Amino Acid Chelated Micronutrients
Amino acid chelates are especially suitable for greenhouse and hydroponics systems because they are usually certified organic, readily available for uptake by plants by both roots and foliage, and are generally not phytotoxic.
For example, in aquaponic systems where fish are integrated into the hydroponics system it is important that nothing synthetic enter the tissues or meat of the fish. Tilapia accumulate iron in the liver where it can become toxic. In aquaponic systems tilapia are fed feed that does not contain iron. Consequently the feces do not contain iron. Therefore, the water coming from the fish tanks does not contain iron and the plants downstream will exhibit iron deficiency, especially in the leafy vegetables like lettuce and basil. Cornell University recommends amino acid chelated iron be injected into the system after the fish tank where it will be absorbed by plant roots before the clean water is returned to the fish side of the system. Therefore, the use of organic materials is an obvious choice, and the amino acid chelates can be applied directly to the foliage or to the nutrient solution for immediate correction of nutrient deficiencies.
Using Iron in Soil, Hydroponics & Aquaponics


 Video Library
Video Library 




















